Pseudomonas syringae on Beet and Swiss Chard
Bacterial leaf spot (BLS), caused by Pseudomonas syringae pv. aptata and related pathogens, can cause losses in table beet (Beta vulgaris subsp. vulgaris), sugar beet (Beta vulgaris subsp. saccharifera), and Swiss chard (Beta vulgaris subsp. cicla) (Jacobsen 2009; Koike et al. 2003; Nampijja et al. 2021b; Stojsin et al. 2015). The disease has been reported from countries in Asia and Europe, as well as Russia, Australia, New Zealand, and the USA.
The impact of BLS on beet and chard production can range from insignificant to complete loss of a crop. For example, in New York State in 2017, BLS was observed in 75% of the table beet crops surveyed, at an average incidence of 80% in these crops grown for harvest of the beet roots. In such crops, BLS can significantly reduce the amount of healthy foliage available for mechanized beet harvest by top-pulling machinery. At the end of the season, severe damage can result in a complete loss if beets are not pulled due to the foliage detaching, especially in waterlogged or rocky soils in late fall harvests.
In baby leaf beet and chard crops, BLS can result in rejection of entire crops because of the appearance of symptomatic leaves (Derie et al. 2016; Koike et al. 2003; Nampijja et al. 2021a, 2021b). In seasons with adequate supply of product, a baby leaf beet or chard crop may be rejected if >5% of the leaves have symptoms of BLS. The risk of BLS is very high in baby leaf crops because of the extremely dense seeding rates (up to 9 million seed/ha), frequent overhead irrigation, and very short duration to harvest with sequential plantings. In addition, up to 40% of the baby leaf beet and chard crops grown in the USA are certified organic, which limits the choice of products available to help suppress BLS.
BLS is common in many sugar beet production areas but typically does not cause economically significant losses (Jacobsen 2007). However, severe losses to BLS in sugar beet have been documented. For example, in 2013, BLS was observed in 25 commercial sugar beet fields in the Vojvodina Province of Serbia, at a range of 0.1 to 40% severity in these fields (Stojsin et al. 2015). Serbia is a major sugar beet production area in southeastern Europe. Sugar beet crops are dug, unlike many table beet root crops that are harvested by the tops, so the impact of BLS typically is less in sugar beet crops, but severe foliar infections can reduce photosynthesis and, therefore, root size.
BLS symptoms typically have not been observed readily in beet and chard seed crops, but detection of the pathogens on beet and chard seed has been increasing for seed lots grown in the primary regions of seed production in the world, including the Pacific Northwest USA (western Washington and Oregon), New Zealand, South Africa, Europe (Safni et al. 2016; du Toit and Bull, unpublished). During the 2019-2020, biennial season for production of table beet and Swiss chard seed, an extended period of cool and wet weather from spring through early July 2020 was associated with a very significant increase in prevalence of BLS symptoms in seed crops surveyed in western Washington. In comparison, a similar survey of a dozen beet and chard seed crops in 2015, one of the warmest and driest seasons on record for western Washington, failed to identify any BLS symptoms, and the BLS pathogens could not be isolated from any of the samples collected.
Seedborne infection by the BLS pathogens results in an important inoculum source for disseminating pathogens and establishing infection in the crops grown from infected seed lots. Planting infested seed can result in 100% crop loss under favorable conditions for the pathogen.
Hosts
Table beet (Beta vulgaris subsp. vulgaris), sugar beet (Beta vulgaris subsp. saccharifera), and Swiss chard (Beta vulgaris subsp. cicla) are susceptible to a wide variety of genotypes of Pseudomonas syringae pv. aptata that have been isolated from symptomatic beet and chard plants. Strains may differ in virulence on table beet and chard. Some strains have only caused symptoms on table beet, and others have caused symptoms on both beet and chard (Derie et al. 2016; Safni et al. 2016). It remains to be determined whether these pathogens are also pathogenic on cucurbits, but there are reports of P. syringae pv. aptata and related pathogens causing diseases on cucurbits (Morris et al. 2000), though the strains may not be identical to those causing BLS on beet and chard. There is preliminary evidence that strains of P. syringae pv. aptata pathogenic on beet and chard can cause a leaf spot on common lambsquarters, Chenopodium alba, a common Chenopodiaceae weed in many areas of beet and chard production.
Symptoms
Symptoms can develop in beet and chard food crops (root crops, baby leaf crops, and mature leaf crops), in sugar beet crops, in fodder beet crops (for animal feed), and in biennial seed crops of any of these species (Jacobsen 2007; Khan et al. 2003; Koike et al 2003; Nampijja et al. 2021; Stojsin et al. 2015).
- Seedlings that develop from infected seed may have spots on the cotyledons, and cotyledons may be distorted from expanding around dead, infected tissue.
- Severely infected seedlings may die.
- Dark brown to black leaf spots or streaks can develop on leaves. Leaf spots are initially 2 to 3 mm in diameter, and develop a distinct, narrow, dark brown to black margin. Leaf spots readily coalesce under favorable conditions to cause blighting of leaves.
- The pathogens can enter leaves through stomata, resulting in leaf spots; through hydathodes (natural openings where veins reach the end of the leaf), leading to necrotic/black lesions on the margins of leaves; and through wounds (mechanical, insect feeding, or chemical), leading to irregular lesions associated with the site of infection at the damaged tissue.
- Under wet conditions, the leaf spots take on a greasy, water-soaked appearance. Under dry conditions, the leaf spots become dry, tan to brown in color, and develop a distinct thin brown to black margin. Dry leaf spots can be confused with other leaf spot diseases such as Cercospora leaf spot and Phoma leaf spot (Khan et al. 2003).
- Leaves infected when immature often become deformed as they expand as a result of the leaf not being able to develop normally where tissue has been killed by BLS.
- Extensive leaf spotting can reduce the photosynthetic leaf area.
- During the reproductive (second) season of biennial seed crops, necrotic spots can develop on seed stalks.
- Infected flowers may abort, and infection of seed early in maturation can lead to poorly sized seed or aborted seed, but there are usually no symptoms on seed that meets marketable size categories.
 Fig. 1: Bacterial leaf spot caused by Pseudomonas syringae in red beet seed crops. A) Severe, coalesced lesions on a leaf. B) Bacterial leaf spot symptoms on leaves and seed stalks (black arrow) in a table beet seed crop. C) Bacterial leaf spot symptoms on beet seedlings. D) Dark brown to black, water-soaked lesions on a beet leaf following a rainstorm.
Fig. 1: Bacterial leaf spot caused by Pseudomonas syringae in red beet seed crops. A) Severe, coalesced lesions on a leaf. B) Bacterial leaf spot symptoms on leaves and seed stalks (black arrow) in a table beet seed crop. C) Bacterial leaf spot symptoms on beet seedlings. D) Dark brown to black, water-soaked lesions on a beet leaf following a rainstorm.

Fig. 2: Bacterial leaf spot in baby leaf Swiss chard crops.
Causal Agents
BLS of beet and chard are caused predominantly by two main genotypes closely related to Pseudomonas syringae pv. aptata and P. syringae pv. atrofaciens within P. syringae sensu stricto (in the strictest sense). Read more about the taxonomy of the pathogens causing these diseases here.
Disease Cycle
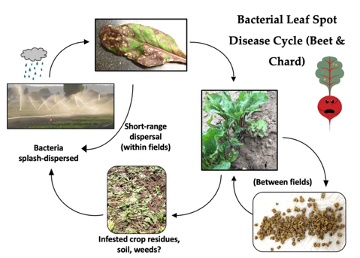
Figure 3: Generalized disease cycle for Pseudomonas syringae on beets and chard.
Epidemiology
The bacteria that cause BLS are readily seedborne and seed transmitted in beet and chard, but there has been limited research on seedborne inoculum thresholds for the disease (Derie et al. 2016). The disease tends to be more severe in ‘baby leaf’ crops as a result of very dense seeding rates, overhead irrigation, and the short duration to harvest (25-40 days from planting to harvest) with adjacent, sequential plantings over an extended period. Seed transmission has been assessed in a series of field experiments (Derie et al. 2016; Crane et al. research in progress). The BLS pathogens are splash-dispersed by rain and overhead irrigation. The bacteria can exist epiphtyically on leaves without causing symptoms until conditions are favorable and the pathogen population has increased considerably, making it difficult to know when the bacteria might have established in a crop. The bacteria can enter leaves through stomata, hydathodes, and wounds from mechanical injury, insect feeding, or chemical damage. BLS is most prevalent and severe during wet and cool to warm (10-25°C) conditions, although the bacteria have been documented to grow at a temperature range of 2 to 35°C. Besides seedborne inoculum, the pathogens can survive in surface irrigation water, which can contribute to outbreaks of BLS. In addition, partially buried, infected beet and chard crop residues from previous crops that remain exposed on the soil surface can be a source of inoculum.
Management
Effective management of BLS necessitates an integrated management program that includes cultural practices and, potentially, application of bactericides (Franc et al. 2001; Jacobsen 2007; Nampijja et al. 2021b; Nikolić et al. 2018a). Since there are no systemic, highly effective bactericides for control of BLS, cultural practices are extremely important for limiting losses to BLS. There are currently no highly resistant beet and chard cultivars available commercially.
More information is available on the Pacific Northwest Pest Management Handbook’s website.
Cultural Control
- Crop rotation with non-host plants of P. syringae pv. aptata can be very effective for managing BLS.
- Management of weeds, particularly Chenopod weeds such as common lambsquarters (Chenopodium alba) can limit the risk of weeds serving as reservoirs of inoculum.
- Plant seed lots that have tested negative for the BLS pathogens or seed lots that have been treated to eliminate the pathogen. Several proprietary seed treatments are available that are highly effective for treating infected seed lots.
- There are no cultivars with complete resistance to BLS but there is variation in susceptibility among cultivars. Efforts are in progress to breed for improved resistance to BLS in table beet and Swiss chard.
- Where possible, using drip or furrow irrigation can severely limit splash dispersal of the pathogen.
- If overhead irrigation is used, minimizing the duration the crop canopy remains wet can limit development of BLS, e.g., by irrigating in the mornings so the canopy is not wet through the night, or irrigating for longer durations but less frequently in order for the canopy to dry out between irrigations.
- Contaminated surface irrigation water can be a reservoir of inoculum. Irrigation water can be tested for the BLS pathogens. Infected sources of water may need to be treated with a disinfectant.
- Bury infected crop residues in the soil after harvest to speed up the decomposition of the pathogens in crop residues. Plant tissues break down more rapidly when incorporated into the soil than when left on the soil surface.
- In areas of biennial seed production, avoid planting a new seed crop in close proximity to a seed crop in the second year of production, to reduce the risk of the pathogen spreading between fields.
- Sequential, adjacent plantings within a field of fresh market, root, or processing crops increase the risk of spread of the pathogen because of the green bridge effect between plantings.
Chemical Control
There are no systemic, highly effective bactericides for control of BLS of beet and chard.
- Copper formulations (FRAC Group M1) offer limited control. Numerous copper products are registered for use on beet and chard for various diseases, and some can be applied on beet and chard in some states and countries for control of BLS. Copper formulations must be used preventatively because they do not eradicate bacterial leaf spot once the disease has developed, but can slow progress of the disease. Examples of products include:
- Champ Formula 2 Flowable, Champ WG, Badge SC (copper hydroxide + copper oxychloride), Kocide 2000 or Kocide 3000 (copper hydroxide), MasterCop (copper sulfate pentahydrate), Nordox 75WG Wettable Granule (cuprous oxide), and Nu-Cop 50 DF. Several of these products are approved for use in certified organic crops.
- Oxidate 5.0 (hydrogen peroxide + peroxyacetic acid) is registered for other diseases on beet/chard in some crops at a 1:800 to 1:500 dilution, applied on a 5- to 10-day interval for preventative application. There is limited evidence that applications of this product provide effective control of BLS.
Biological Control
Although a number of biological control products are registered for use in beet for control of bacterial diseases, the efficacy of these products for control of bacterial leaf spot is unknown. Many have not proven effective. Examples include Double Nickel LC (FRAC Group 44), and LifeGard WG (FRAC Group P6) for activating plant resistance.
Nikolić et al. (2018a) demonstrated significant biocontrol potential of crude lipopeptide extracts (CLE) and cell cultures of the bacterium Bacillus amyloliquefaciens SS-12.6, which successfully suppressed BLS when tested on sugar beet plants.
Project Goals Related to Chenopods
- Objective 1
Develop diagnostic methods for detection and quantification of the pathogens in seed and from environmental inoculum sources. - Objective 2
Development of novel IPM practices for crop production and seed production to reduce seed contamination/infection and disease. - Objective 3
Develop seed testing protocols and treatments for quality assurance. - Objective 4
Identifying novel sources of disease resistance to P. syringae chenopod pathogens. - Objective 5
Analyze the cost-effectiveness for all practices developed. - Objective 6
International seed health extension, training, and mentorship.


